Electron Dot Structure Of Co2. A dot is placed around the atom symbol for each valence electron. Lewis dot structures, electron dot structures, or lewis electron dot structures (leds), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule.[1][2][3] a lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds.
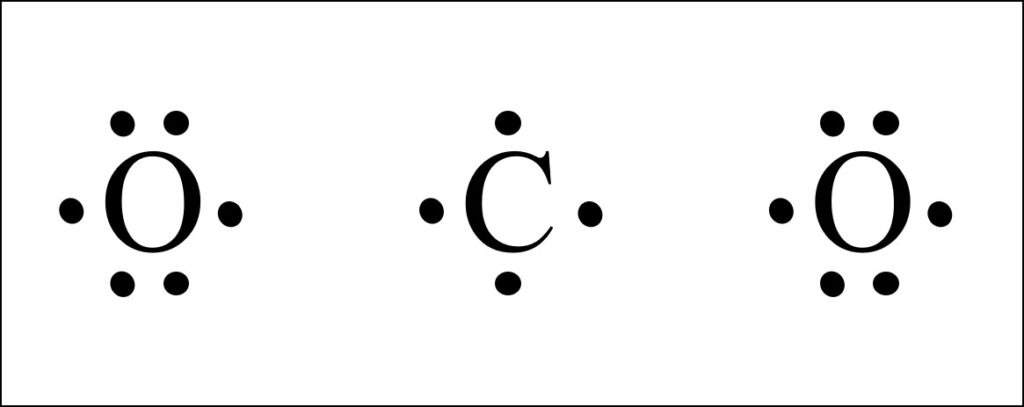
So this is the correct lewis structure for co2. Co2 is a trace gas comprising 0.039% of the atmosphere. Hydrogen has one (1) while chlorine has seven electrons in the valence shell (2, 8, 7).
In The First Step, We Need To Calculate How Many Valence Electrons Are Present.
Carbon atom has four valence electrons (2, 4) ; I quickly take you through how to draw the lewis structure of co2 (carbon dioxide). Before we discuss the co 2 lewis structure or lewis dot structure for co2, we need to know the basics of lewis dot structure.lewis dot structure work on the octet rule, which means that all the atoms in the structure would have eight electrons in.
Find The Least Electronegative Atom And Placed It At Center.
Electrons are placed in the 4 corners of the symbol in order, starting from anywhere. Carbon dioxide (co2) lewis structure has two oxygen atoms and one carbon atom. In step 2 the number of electrons needed to create a bond was determined, while in step 1 the number of electrons present in.
It Is A Gas At Standard Temperature And Pressure And Exists In Earth’s Atmosphere In This State.
So carbon is shown with four dots around it. The total valence electron is available for drawing the sodium chloride (nacl) lewis structure is 8. Since it is bonded to only one carbon atom, it must form a double bond.
Electrons Are Placed Up To Two On Each Side Of The Elemental Symbol For A Maximum Of Eight, Which Is The Number Of Electrons In A Filled S And P Shell.
Write electron dot structure of co2 and alcl3 a coordinate bond (also called a dative covalent bond) is a covalent bond (a shared pair of electrons) in which both electrons come from the same atom. Since it is bonded to only one carbon atom, it must form a double bond. Electron dot structures of carbon dioxide oxygen atom contains 6 valence electrons which form 2 lone pairs.
Lewis Dot Structure Is A Pictorial Representation Of The Arrangement Of The Valence Shell Electrons In The Molecule.
It determines the number of outermost valence electrons as well as the electrons engaged in the co2 molecule’s bond formation. Oxygen atom contains 6 valence electrons which form 2 lone pairs. Now, each atom in our structure has a full octet.
Related Posts
- Carbonate Electron GeometryCarbonate Electron Geometry. Provided to together a skeletal diagram where we use the signs of the atoms and use dots to represent the valence shell ...
- H2O Electron Dot StructureH2O Electron Dot Structure. The o in h2o has 2 bond pairs and 2 lone pairs (again, 4 total pairs ). They also display the total number of lone pairs ...
- Lewis Structure For Bicarbonate IonLewis Structure For Bicarbonate Ion. It consists of one central carbon atom surrounded by three oxygen atoms in a trigonal planar arrangement, with a ...
- 03 Lewis Dot Structure03 Lewis Dot Structure. Let’s see how to draw lewis dot structure for nf3 with easy steps. Nocl, cf 2 cl 2, hcn;03 Lewis Structure from divinewsmedia ...
- Cr 3 Electron ConfigurationCr 3 Electron Configuration. Cr3+ has 3 electrons removed from the outermost shell. 1s 2 2s 2 2p 1:TanabeSugano diagram for the 3d 3 electronic from ...
- S3 Lewis StructureS3 Lewis Structure. When we are done adding valence electrons we check each atom to see if it has an octet (full outer shell). Only negative charges ...
- Scl4F2 Lewis StructureScl4F2 Lewis Structure. Step 1 − step 3. Determine the electron geometry and molecular geometry of sif4.SCl4 Lewis Structure How to Draw the Lewis St ...


