Carbonate Electron Geometry. Provided to together a skeletal diagram where we use the signs of the atoms and use dots to represent the valence shell electrons. ^^ electron pair geometry and molecular geometry won't be the same if there are lone pairs involved.
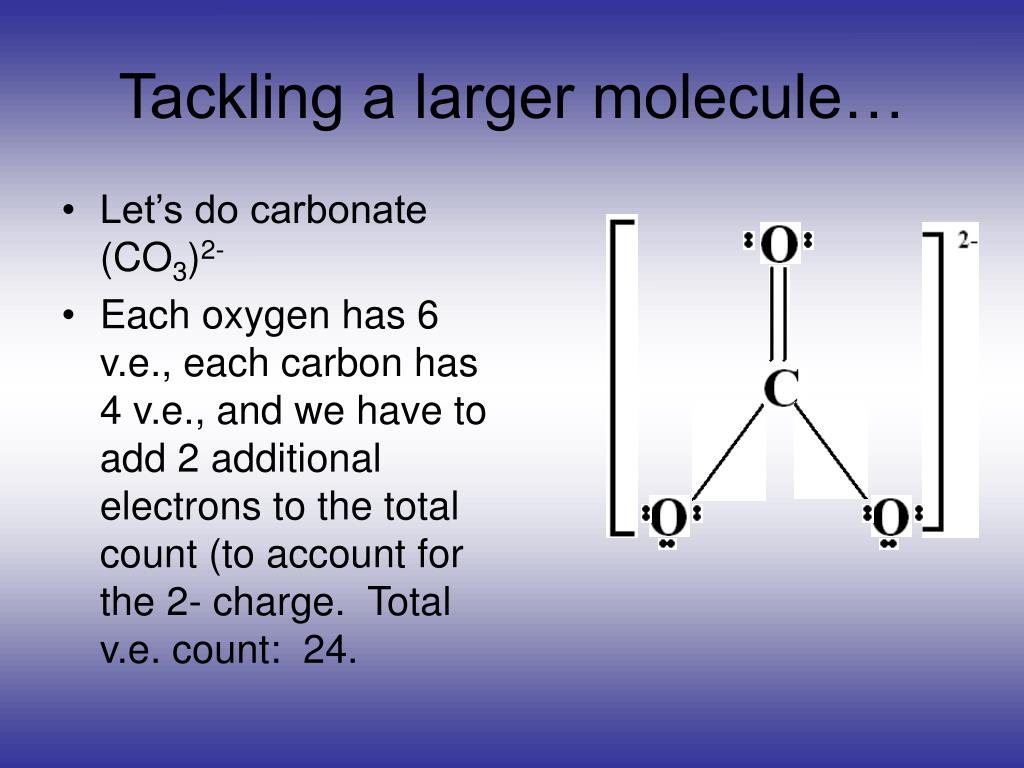
The oxygens with the single bonds have three lone pairs each and the oxygen with the double bond has two lone pairs. Electron geometry single bond lone molecular geometry pairs real molecule formula model bond angle real bond model what is the molecular. The carbon and oxygen are bonded through a double bond which counts as one electron pair and two single bonded
The Number Of Hybrid Orbitals Is Equal To The Number Of Pure Orbitals Involved In The Hybridization.
The two hydrogen atoms from single bonds in. The atom must be excited. (a) mixed oxygen and carbonate ion conducting membranes (mocc), (b) mixed electron and carbonate ion conducting membranes (mecc), and (c) mixed electron, oxygen, and carbonate ion conducting membranes (meocc) [ 16 ].
Molecular Geometry Is Trigonal Planar.
The oxygens with the single bonds have three lone pairs each and the oxygen with the double bond has two lone pairs. The electron geometry for th. It has six electrons in valence shell.
The Orbitals Must Be Close In Energy, Such As 2S With 2P Or 4S With 3D.
The carbon atom attains a stable electronic configuration with eight electrons at the outermost shell. Molecular geometry is trigonal planar. After we write the formula down we use a periodic table or our previous knowledge on the amount of valence electrons for the elements we use.
Thus, The Bond Angle Of Sio2 Is 180O.
^^ electron pair geometry and molecular geometry won't be the same if there are lone pairs involved. (3pts) edit view insert format tools table 12pt paragraph v β ι ο av av t²v. I’m now going to demonstrate how to bond carbonate and what is its molecular geometry.
The Electron Geometry Of Co2 Is Also Linear Because There Is No Lone Pair Present On The Central Atom That Can Cause Disorientation In The Molecule.
Hybridization occurs in the same single atom and produces orbitals that are equivalent in shape, length and energy. Provided to together a skeletal diagram where we use the signs of the atoms and use dots to represent the valence shell electrons. The substance with the chemical formula co 3 goes by the name carbonate.
Related Posts
- Negative Electron Affinity ChartNegative Electron Affinity Chart. This process creates a negative ion. Electron affinity of hydrogen (h) 72.78:Periodicity A couple more things Adria ...
- Electron Dot Structure Of Co2Electron Dot Structure Of Co2. A dot is placed around the atom symbol for each valence electron. Lewis dot structures, electron dot structures, or le ...
- Ground State Electron Configuration For GalliumGround State Electron Configuration For Gallium. Home › gallium ground state condensed electron configuration. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p ...
- Halogen With The Least Negative Electron AffinityHalogen With The Least Negative Electron Affinity. Not all elements form stable negative ions in which case the electron affinity is zero or even pos ...
- H2O Electron Dot StructureH2O Electron Dot Structure. The o in h2o has 2 bond pairs and 2 lone pairs (again, 4 total pairs ). They also display the total number of lone pairs ...
- What Is The Abbreviated Electron Configuration For SilverWhat Is The Abbreviated Electron Configuration For Silver. Now, find the atomic number of the first. The energy of atomic orbitals increases as the p ...
- Cr 3 Electron ConfigurationCr 3 Electron Configuration. Cr3+ has 3 electrons removed from the outermost shell. 1s 2 2s 2 2p 1:TanabeSugano diagram for the 3d 3 electronic from ...




