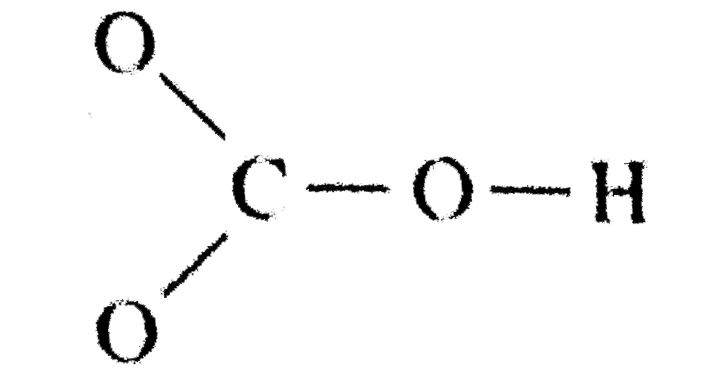
Lewis Structure For Bicarbonate Ion. It consists of one central carbon atom surrounded by three oxygen atoms in a trigonal planar arrangement, with a. Therefore it is put in the center of the dot structure.
Ion formation increases plasma bicarbonate and buffers excess hydrogen ion concentration resulting in. Na becomes na+ by losing one electron from its valence shell and getting an overall positive charge. What is the formula for na2co3?
Hence, The Lewis Symbol For The Sodium Ion Does Not Have A Dot, But A Plus Sign As A Superscript.
What is the lewis symbol for na+? Resonance occurs when two or more valid lewis structures may be How to draw the lewis structure for check me out:
The Dot Structure For Io4 Starts With The I Atom In The Center.from This, There Are Three Doubly Bonded O Atoms And One Singlybonded O Atom.
Determine the formal charge of each atom. The ion for which the lewis structure is to be drawn is bicarbonate ion. What is the lewis symbol of sodium?
Since Carbon Is Located In Period 2 It Does Not Have Access To The D Sublevel And Must Adhere To The Octet Rule.
Hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. This structure is incompatible with the observed symmetry of the ion which implies that the three bonds are equally long and that the three oxygen atoms are equivalent. To keep watching this video solution for free, download our app.
Nci Thesaurus (Ncit) Hydrogencarbonate Is The Carbon Oxoanion Resulting From The Removal Of A Proton From Carbonic Acid.
Chemical bondingbook:r sharmaboard:iit jeeyou can ask any doubt. Ion formation increases plasma bicarbonate and buffers excess hydrogen ion concentration resulting in. It is a polyatomic anion with the.
Join The 2 Crores+ Student Community Now!
What is the formula for na2co3? This browser does not support the video element. Na becomes na+ by losing one electron from its valence shell and getting an overall positive charge.
Related Posts
- Cacl2 Lewis StructureCacl2 Lewis Structure. The only thing you could be doing is the lewis structures of the individual ions formed by atoms, but there would not any cova ...
- Structure Of Hclo3Structure Of Hclo3. Remember, hydrogen always goes on the outside. The h atom would actually be bonded to one of the oxygen atoms that is connected t ...
- What Is The Lewis Dot Structure For Nh3What Is The Lewis Dot Structure For Nh3. Lewis dot diagram of nh3 the lewis structure of ammonia, nh3, would be three hydrogen atoms bonded to a nitr ...
- Ch3Nh2 Lewis Structure Molecular GeometryCh3Nh2 Lewis Structure Molecular Geometry. 2) label every central atom with a molecular geometry and a hybridization. And for this nitrogen is tribun ...
- Lewis Structure Of Sulfur TrioxideLewis Structure Of Sulfur Trioxide. Drawing so3 lewis structure is very easy to by using the following method. So 3 has 24 valence electrons.ArchivoS ...
- Sef2 Lewis Dot StructureSef2 Lewis Dot Structure. It is important to look at what the lewis structure of sf2 is so that we can move ahead and look at other aspects of it. Th ...
- Scl4F2 Lewis StructureScl4F2 Lewis Structure. Step 1 − step 3. Determine the electron geometry and molecular geometry of sif4.SCl4 Lewis Structure How to Draw the Lewis St ...




