How To Determine Whether A Molecule Is Polar. Do check with your school. If a molecule has a net dipole moment (sum of all the dipole moment values of all the polar bonds in it), the molecule is said to be a polar molecule.
Recorded on march 5, 2012 using a flip cam. Polar molecules occur when there is an electronegativity difference between the bonded atoms. Determine if the bonds are ionic or covalent.
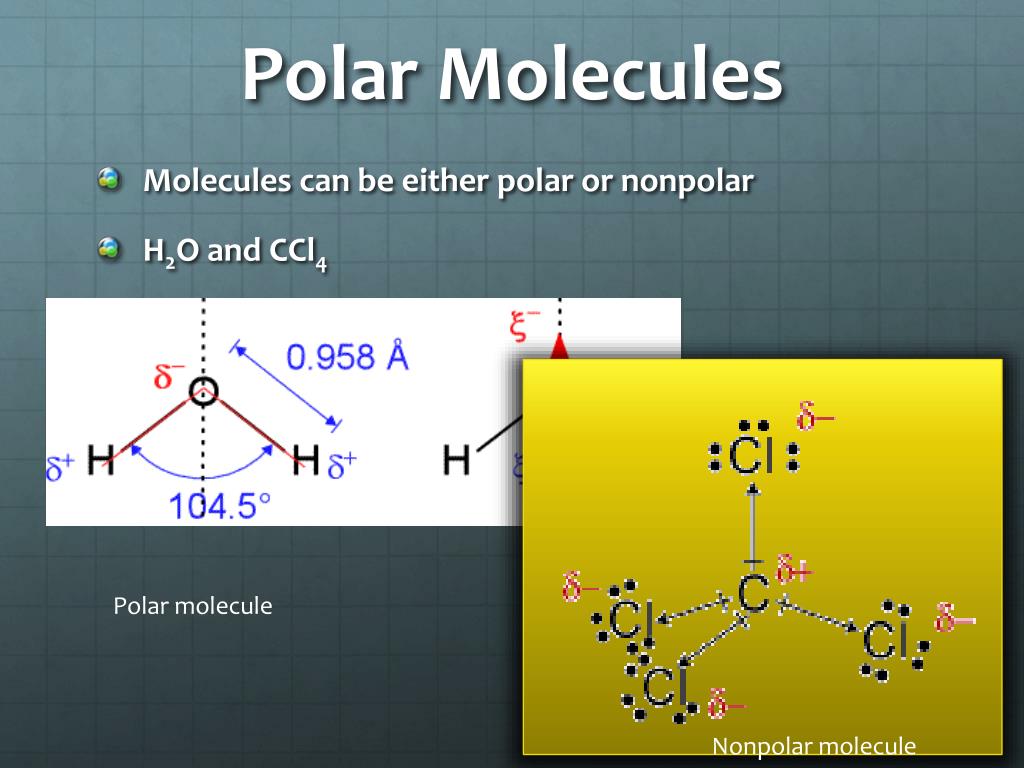
The Bonds Cancel Each Other Out, Are Symmetrical, And There’s No Lone Electron Pair.
Dipole moment is a vector quantity (it has direction). If a molecule has a net dipole moment (sum of all the dipole moment values of all the polar bonds in it), the molecule is said to be a polar molecule. This problem has been solved!
When Two Bonded Atoms Exchange Electrons In An Unequal Manner, Polar Bonds Form.
This question can be easily answered by looking at the t. Figure out the geometry (using vsepr theory) visualize or draw the geometry. There are many things that determine whether something is polar or nonpolar, such as the chemical structure of the molecule.
So The Other Question Is, How Do You Figure Out Whether Or Not It's Polar Or Not?
How do you determine whether a molecule is polar? What makes a molecule polar? If the molecule is perfectly symmetric, the molecule will not be polar even if there are polar bonds present.
How Do You Determine Whether A Molecule Is Polar Or Not?
How to determine if a molecule is polar or not? Another factor that determines the polarity of the molecule is the geometry of the molecule. Determine whether the molecule contains polar bonds.
The Bonds Don’t Cancel Each Other Out And Are Asymmetrical.
To determine whether a bond is polar, you look at the electronegativity difference between the atoms. Draw the lewis structure for the molecule and determine the molecular geometry. How do you determine whether a molecule is polar?
Related Posts
- Is Kcl PolarIs Kcl Polar. The shape of kcl is linear. Question = is nacl polar or nonpolar ?Dispersion and Polar Component of Specific Surface Free from file.sci ...
- Bcl3 Polar Or NonpolarBcl3 Polar Or Nonpolar. Therefore this molecule is nonpolar. The three chloride atoms have a negative charge, and the one boron in the.Is BCl3 a nonp ...
- Brcl5 Polar Or NonpolarBrcl5 Polar Or Nonpolar. The molecular geometry of brf5 is sq. Is bcl3 polar or nonpolar?boron trichloride, or bcl3, is nonpolar.the three chloride a ...
- Xeo3 PolarXeo3 Polar. Because of the presence of one lone pair, its geometrical shape is trigonal pyramidal. Question = is clf polar or nonpolar ?xeo3 polar or ...
- Determine The Name For Tico3 Remember That Titanium Forms Several IonsDetermine The Name For Tico3 Remember That Titanium Forms Several Ions. 7) determine the name for tico3. Remember that co forms several ions.Solved C ...
- Why Is Water Considered PolarWhy Is Water Considered Polar. First, it could be due to the equal sharing of electrons between the atoms. The unequal sharing leads to polarity of w ...
- Polar Or Nonpolar Nh3Polar Or Nonpolar Nh3. Ammonia with a chemical formula of nh3 is a polar. The lone pair electrons) and a region of relative positive charge by the hy ...


