Brcl5 Polar Or Nonpolar. The molecular geometry of brf5 is sq. Is bcl3 polar or nonpolar?boron trichloride, or bcl3, is nonpolar.the three chloride atoms have a negative charge, and the one boron in the center has an equal but positive charge.
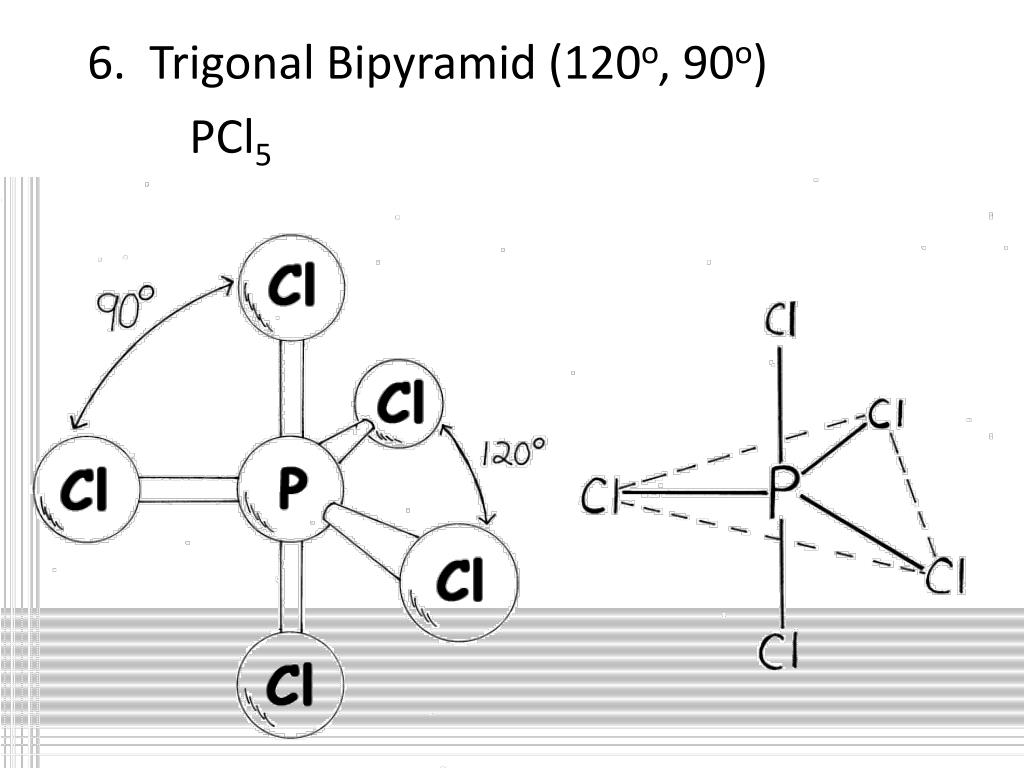
Brcl 5 (bromine pentachloride) is sometimes called a polar molecule, sometimes called nonpolar. Due to low difference of electronegativities, brcl₅ is a nonpolar molecule.; For brcl, the difference is only 0.2 so it is classified as a nonpolar molecule.
Polar Molecules Must Contain Polar Bonds Due To A Difference In Electronegativity Between The Bonded Atoms.
¿el hcl es polar o no polar? Criminal law problem question model answer manslaughter; Classify each of the molecules given below as polar or nonpolar.
Is Brcl5 Polar Or Nonpolar Kategori Produk.
I would have to say that the largest molecule of this group is brcl5. What is the hybridization of the central atom in the lewis structure for brcl5? Similarly, what is the molecular geometry of xef5+?
Brcl5 (Bromine Pentachloride) Is Usually Referred To As A Polar Molecule, Generally Referred To As Nonpolar.
As we can see that the xef4 molecular geometry has the symmetric distribution of electrons and they make a formation in the single square plane. ¿el brcl5 es polar o no polar? Brcl 5 (bromine pentachloride) is sometimes called a polar molecule, sometimes called nonpolar.
The Lewis Structure (Lewis Dot Diagram) For If5.1.
Boron sits in the center of the molecule and has three valence electrons, so it balances out the three chlorides. Although the bonds themselves are polar, the four bonds between carbon and fluorine cancel out one another, generating a nonpolar molecule. Brcl5 is an interhalogen compound and consists of one iodine atom and five chlorine atoms.
Polar In Chemistry, Polarity Is A Separation Of Electric Charge Leading To A Molecule Or Its Chemical Groups Having An Electric Dipole Or Multipole Moment.
A nonpolar solvent will dissolve in a nonpolar solvent but not a polar solvent. Which one is it really and why? Similarly, you may ask, is xef4 polar or nonpolar?
Related Posts
- Is Pbr5 PolarIs Pbr5 Polar. Laboratory chemical safety summary (lcss) datasheet. (phosphorous pentafluoride) if playback doesn't begin shortly, try restartin ...
- Is Kcl PolarIs Kcl Polar. The shape of kcl is linear. Question = is nacl polar or nonpolar ?Dispersion and Polar Component of Specific Surface Free from file.sci ...
- Is F2 Polar Or NonpolarIs F2 Polar Or Nonpolar. Is f2 a polar or nonpolar bond? Polar molecules must contain polar bonds due to a difference in electronegativity between th ...
- Why Is Water Considered PolarWhy Is Water Considered Polar. First, it could be due to the equal sharing of electrons between the atoms. The unequal sharing leads to polarity of w ...
- How To Determine Whether A Molecule Is PolarHow To Determine Whether A Molecule Is Polar. Do check with your school. If a molecule has a net dipole moment (sum of all the dipole moment values o ...
- Bcl3 Polar Or NonpolarBcl3 Polar Or Nonpolar. Therefore this molecule is nonpolar. The three chloride atoms have a negative charge, and the one boron in the.Is BCl3 a nonp ...
- Polar Opposite SynonymsPolar Opposite Synonyms. Opposite , polar opposite , antithetical , counter , conflicting , different , contradictory , clashing , opposing , opposed ...



