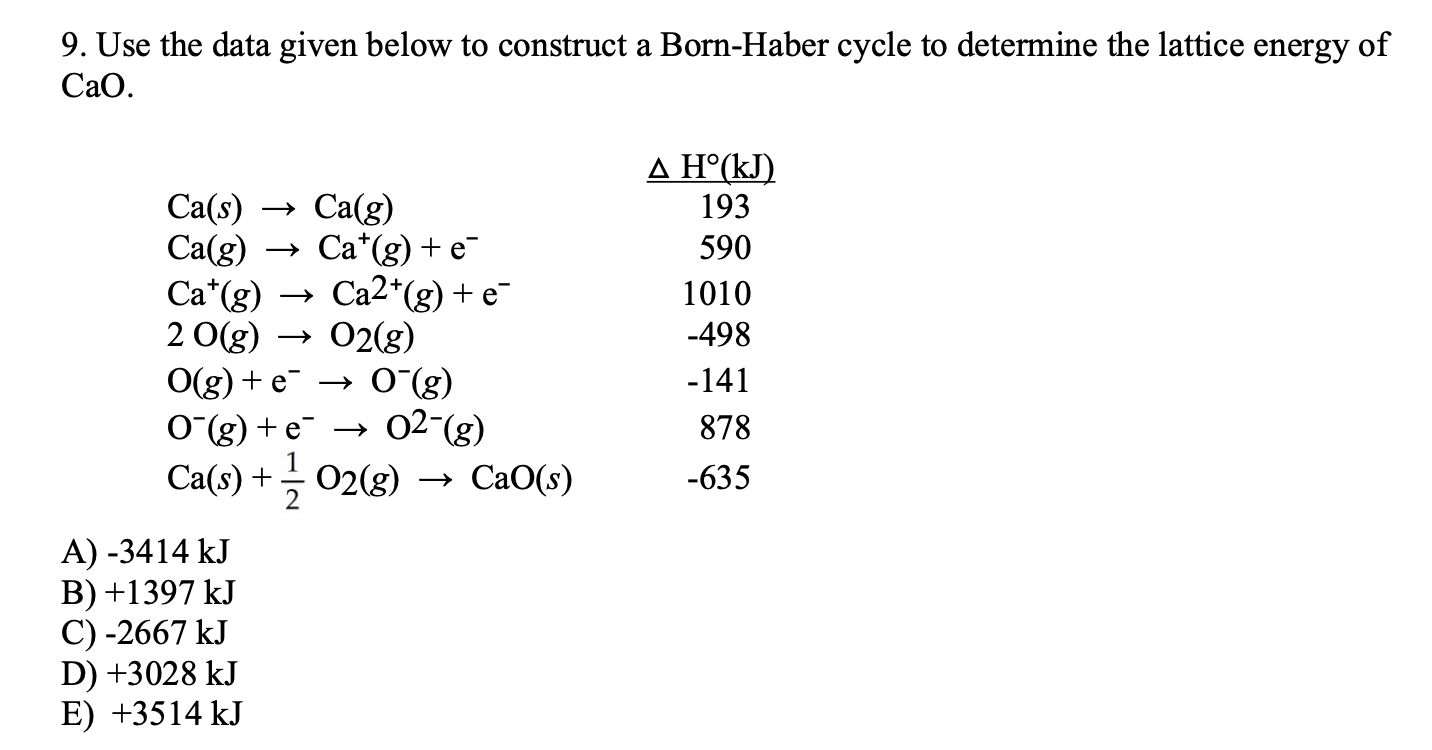
Use The Data Given Below To Construct A Born Haber Cycle To Determine The Lattice Energy Of Cao. This question is asked that from the following data. Hi, could someone please take a look at the following question?
We have a complete cycle. Identify the bond with the highest bond energy. All right for this problem.
For The Reaction To Proceed, K Is To Be Converted To The Gaseous State.
This question is asked that from the following data. Therefore, we have this equation: Recall that lattice energy is the energy required to combine two gaseous ions into a solid ionic compound:.
Use The Following Data To Calculate The Lattice Energy Of Calcium Oxide.
So the 1st 1 i have is calcium solid to calcium gas, and that was 178 killer jules promote. Give the complete electronic configuration for ca 2+. Ca(s) + ½ o2 (g) → cao(s) let us look at the data provided:
In A Cycle, The Net Energy Of The Reaction Can Be Calculated.
We’re being asked to calculate the 2nd ionization energy of calcium (ca) using the provided transformation. We start with the corresponding formation equation. Take the δh ∘ f step as being upwards to generate a complete cycle, for which δh cycle = 0 (since h f = h i for a complete cycle).
Dh°(Kj) Ca(S) → Ca(G) 193 Ca(G) → Ca⁺(G) + E⁻ 590 Ca⁺(G) Biology.
Use a born haber cycle to calculate the lattice energy (in kj/mol) of potassium chloride (kcl) from the following data: 100% (5 ratings) transcribed image text : A) lattice energy b) electron affinity c) heat of formation 3.
Then I Have Oxygen Gas Or 1/2 Oh To To A Single Oxygen Molecule, Which Was 249 Point To Kill Jules.
We are forming cao from ca(s) and o2 (g). We have a complete cycle. Ah (kj/mo) sublimation of calcium 193 first…
Related Posts
- 2 What Is The Information Processing Cycle2 What Is The Information Processing Cycle. If/then (conditionals), finding a match (searching), counting, and comparing. Usually, it's used to ...
- Elements With Highest Ionization EnergyElements With Highest Ionization Energy. From this trend, cesium is said to have the lowest ionization energy and fluorine is said to have the highes ...
- Consider The Combination Of Resistors Shown In The Figure BelowConsider The Combination Of Resistors Shown In The Figure Below. Solution for consider the combination of resistors shown in the figure below. Share ...
- Construct A Simulated 1H Nmr Spectrum For The Given Structural FormulaConstruct A Simulated 1H Nmr Spectrum For The Given Structural Formula. Drag the appropriate splitting patterns to the approximate chemical shift pos ...
- Energy Converting OrganellesEnergy Converting Organelles. Energy converting organelles of heterotrophs; This would explain why mitochondria and chloroplasts contain their own dn ...
- What Is The Iupac Name For The Compound Shown Below H3C Ch2 Ch Ch2What Is The Iupac Name For The Compound Shown Below H3C Ch2 Ch Ch2. The iupac name of the compound ch 3. The answer butene would have been wrong if t ...
- Of The Atoms Below Is The Most ElectronegativeOf The Atoms Below Is The Most Electronegative. The most electronegative atom in a compound has a charge that is in general electronegativity is the ...
