Is Pbr5 Polar. Laboratory chemical safety summary (lcss) datasheet. (phosphorous pentafluoride) if playback doesn't begin shortly, try restarting your device.
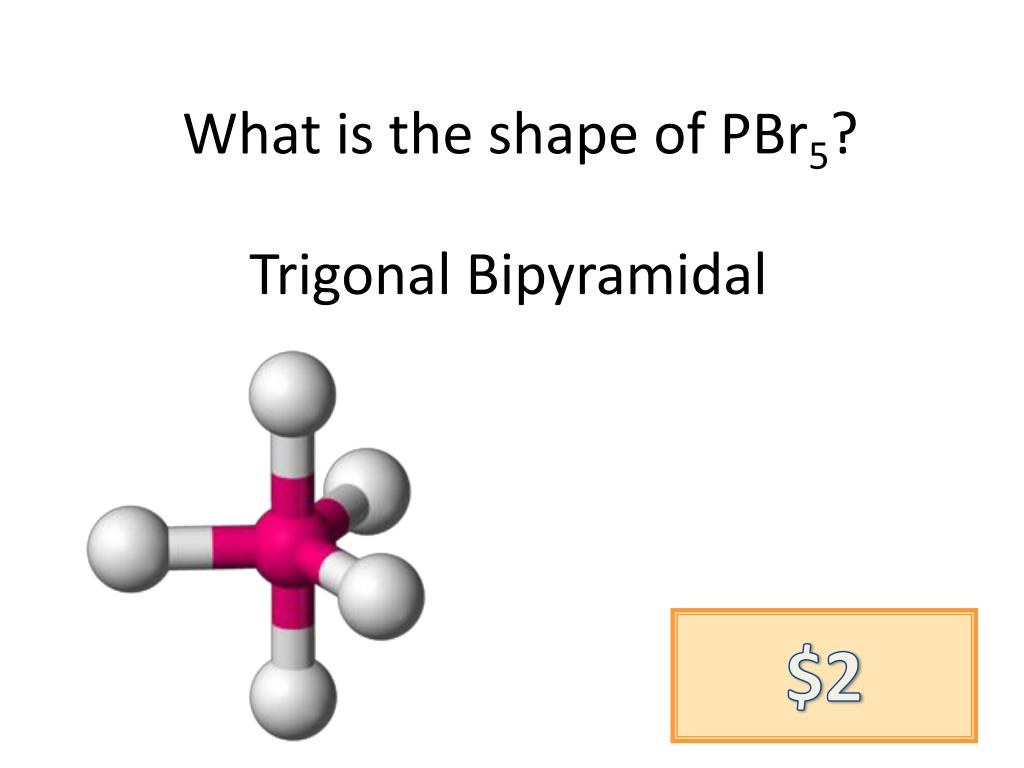
Why is pbr5 considered non polar when its difference in electronegativity is 0.7, which questions chemistry why is pbr5 considered non polar when its difference in electronegativity is 0.7, which would make it polar covalent? E) i, ii, and iii. Answer = pbr5 ( phosphorus pentabromide ) is nonpolar.
In This Video We Are Going To Determine The Polarity Of Phosphorus Pentabromide Having A Chemical Formula Of Pbr5.What Is Polarity?
(phosphorus pentabromide) in this video we are going to determine the polarity of phosphorus pentabromide having a chemical formula of pbr5. Brf5 or bromine pentafluoride is a polar molecule as the molecular geometry of brf5 falls out to be square pyramidal with an asymmetric charge distribution concentrating on the central atom. The molecule contains a central bromine atom which is encompassing a total of five fluorides and forming a lone pair of electrons.
E) I, Ii, And Iii.
Xef4 ccl4 xef2 are non polar while pbr5 and bf5 is polar. Answer = pbr5 ( phosphorus pentabromide ) is nonpolar. Is pbr5 polar or nonpolar?
As A Result Of This Geometry And Arrangement Of Electrons, The Net Dipole Moment Of The Molecule Is Zero.
Xef4 = square planar (ax4e2), ccl4 = tetrahedral (ax4), xef2 = linear (ax2e3), pbr5 = trigonal pyramidal (ax5) each of the above molecules have a symmetrical shape and arrangement of polar bonds. A) nonpolar bonds, and is a nonpolar molecule. Is pbr5 polar or nonpolar?
Br Continue Reading Related Answer
(phosphorous pentafluoride) if playback doesn't begin shortly, try restarting your device. This results in the cancellation of all polar vectors and a net zero dipole moment. This results in the cancellation of all polar vectors and a net zero dipole moment.
Hence There Is No Polarity Observed In The Compound And Pbr5 Is Thus Nonpolar.
Polarity is a term used in electricity, magnetism, and electronic signaling. To conclude all the properties of the molecule pbr5, it can be said that the molecule has 40 valence electrons out of which there are 15 lone pairs of electrons. But due to its asymmetrical shape, it is considered a polar molecule.
Related Posts
- Is F2 Polar Or NonpolarIs F2 Polar Or Nonpolar. Is f2 a polar or nonpolar bond? Polar molecules must contain polar bonds due to a difference in electronegativity between th ...
- Polar Or Nonpolar Nh3Polar Or Nonpolar Nh3. Ammonia with a chemical formula of nh3 is a polar. The lone pair electrons) and a region of relative positive charge by the hy ...
- Is Kcl PolarIs Kcl Polar. The shape of kcl is linear. Question = is nacl polar or nonpolar ?Dispersion and Polar Component of Specific Surface Free from file.sci ...
- Xeo3 PolarXeo3 Polar. Because of the presence of one lone pair, its geometrical shape is trigonal pyramidal. Question = is clf polar or nonpolar ?xeo3 polar or ...
- Brcl5 Polar Or NonpolarBrcl5 Polar Or Nonpolar. The molecular geometry of brf5 is sq. Is bcl3 polar or nonpolar?boron trichloride, or bcl3, is nonpolar.the three chloride a ...
- Why Is Water Considered PolarWhy Is Water Considered Polar. First, it could be due to the equal sharing of electrons between the atoms. The unequal sharing leads to polarity of w ...
- Bcl3 Polar Or NonpolarBcl3 Polar Or Nonpolar. Therefore this molecule is nonpolar. The three chloride atoms have a negative charge, and the one boron in the.Is BCl3 a nonp ...



