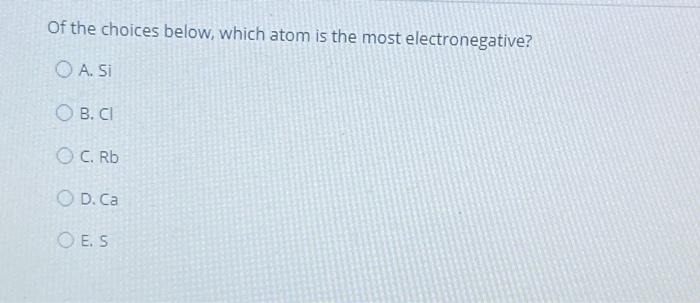
Of The Atoms Below Is The Most Electronegative. The most electronegative atom in a compound has a charge that is in general electronegativity is the measure of an atom's ability to attract electrons to itself in a covalent bond. Which two bonds are most similar in polarity?
This is the most electronegative elements on the periodic table starting with the most electronegative on the top, and decreasing in electronegativity as we work down. 2 🔴 on a question question 8 of the atoms below, is the most electronegative. A n b br c o d f e cl
We Have Beryllium, Magnesium Over Here And Then Carbon And Silicon.
A) br b) o c) cl d) n e) f Therefore, the most electronegative atoms can be found in the upper, right hand side of the periodic table, and the least electronegative elements can be found at the bottom left. So let's see, we have uh this square right here.
The Most Electronegative Element From The Choices Given Is Sulfur (S).
How is prose poetry different from prose? Of the atoms below, _ is the most electronegative. What is the most electronegative atom;
Question 8 Of The Atoms Below, _____Is The Most Electronegative.
Plz help i'll award brinliest. 3 show answers another question on chemistry. You have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide.
A N B Br C O D F E Cl
Be= 1.57, o= 3.44, n= 3.04. I didn't know you were a woman. I am also sorry for calling you sir.
Of The Atoms Below, Is The Least Electronegative.
So we have fluorine, oxygen, nitrogen, chlorine, bromine, iodine, sulfur, carbon, and hydrogen. This is the most electronegative elements on the periodic table starting with the most electronegative on the top, and decreasing in electronegativity as we work down. Differences in element electronegativities may be used to predict the type of bonding, ionic or covalent, in a substance.
Related Posts
- Consider The Combination Of Resistors Shown In The Figure BelowConsider The Combination Of Resistors Shown In The Figure Below. Solution for consider the combination of resistors shown in the figure below. Share ...
- What Is The Iupac Name For The Compound Shown Below H3C Ch2 Ch Ch2What Is The Iupac Name For The Compound Shown Below H3C Ch2 Ch Ch2. The iupac name of the compound ch 3. The answer butene would have been wrong if t ...
- Most Famous Spanish ArtistMost Famous Spanish Artist. Early on in his career, goya was celebrated for his royal portraits and in 1779 he won an appointment as a painter to the ...
- Use The Data Given Below To Construct A Born Haber Cycle To Determine The Lattice Energy Of CaoUse The Data Given Below To Construct A Born Haber Cycle To Determine The Lattice Energy Of Cao. This question is asked that from the following data. ...
