What Is The Change In The Atomic Number When An Atom Emits An Alpha Particle. Alpha decay is a nuclear change process which produces an alpha particle. What is the change in atomic mass number when an atom emits an alpha particle?
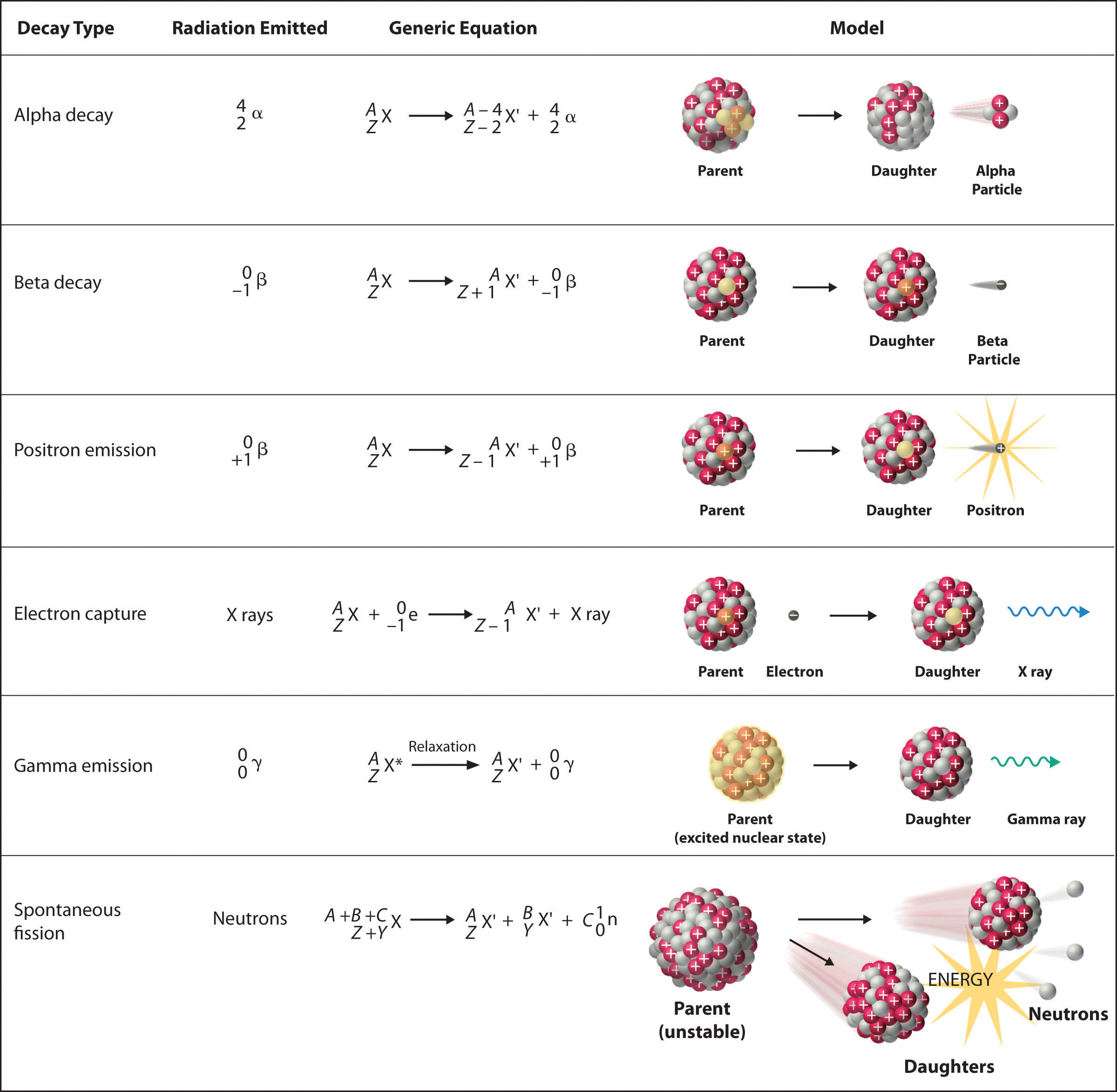
When a nucleus emits an alpha particle, these changes happen: Beta decay is fundamentally different from alpha decay. What is the change in the atomic number when an atom emits an alpha particle?
When An Element Changes From An Unstable Form To A Stable One.
Furthermore, what change in the atomic number. What is the change in the atomic number when an atom emits an alpha particle? If an isotope decays by the process of beta emission, ____.
An Alpha Particle Is Essentially A Helium Nucleus Composed Of Two Protons Andneutrons.
The mass number decreases by 4; This is called alpha decay. However, if an alpha particle is emitted, the atomic number decreases by 2 and becomes 82, and the atomic mass decreases by 4 and drops to 214.
The Atom Will Have Undergone Transmutation Into A Different Element.
Beta decay is fundamentally different from alpha decay. The atomic number decreases by 2 When a nucleus emits an alpha particle, these changes happen:
An Atom Of Fluorine Has An Atomic Number Of 9 And An Atomic Mass Of 19.
Alpha particle is a helium nucleus so it has more power compare to proton. What is the change in atomic mass when an atom emits a beta particle; This is called alpha decay.
What Is The Mass Number Of An Ion With 108 Electrons 157 Neutrons And A +1 Charge Express Your Answer As An Integer?, Answer:
It charge has +2e, which is twice of a proton. Since atomic number is the number of protons, if an atom emits an alpha particle, its atomic number will be reduced by two, and its mass number will be reduced by four. The mass number decreases by 4.
Related Posts
- 2 What Is The Information Processing Cycle2 What Is The Information Processing Cycle. If/then (conditionals), finding a match (searching), counting, and comparing. Usually, it's used to ...
- What Is 1 70 Meters In FeetWhat Is 1 70 Meters In Feet. To convert feet to meters, divide your feet figure by 3.28. How far is 70 meters in feet?Understanding and Dealing with ...
- Prokaryotes Lack WhatProkaryotes Lack What. In addition to the lack of organelles, prokaryotic cells also lack a cytoskeleton. A prokaryotic cell also lacks mitochondria ...
- What Is A Perfect Square Trinomial DefinitionWhat Is A Perfect Square Trinomial Definition. Definition of perfect square trinomial. Math explained in easy language, plus puzzles, games, quizzes, ...
- 1 625 As A Mixed Number1 625 As A Mixed Number. Therefore, 34.625 as a fraction is as follows: It is also equal to 1 9/16 when writen as a mixed number.Rational Numbers Con ...
- How Many Valence Electrons Are In An Atom Of NitrogenHow Many Valence Electrons Are In An Atom Of Nitrogen. The valence level of an atom refers to the number of electrons that reside in the upper most e ...
- What Do You Get When You CrossWhat Do You Get When You Cross. (the outside!) why do hens lay eggs? What do you get when you cross a fly, a car, and a dog?What do you get when you ...

