How Many Valence Electrons Are In An Atom Of Nitrogen. The valence level of an atom refers to the number of electrons that reside in the upper most energy level. Therefore, sulfur has 6 valence electrons.
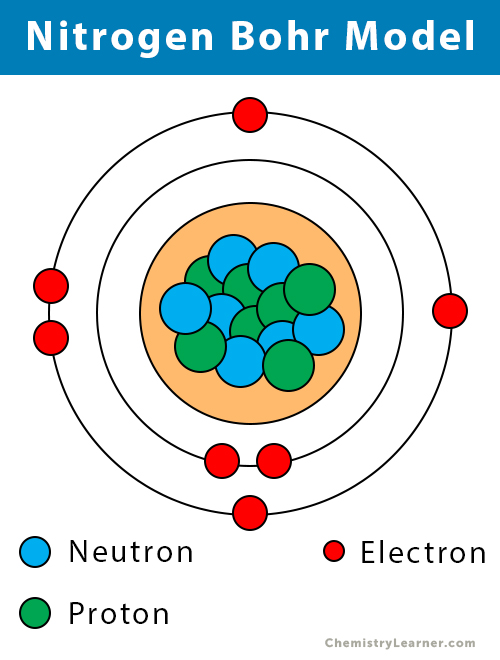
Nitrogen is found to have either 3 or 5 valence electrons and also lies at the optimal of group 15 on the routine table. Asked by wiki @ 01/07/2021 in chemistry viewed by 27 persons. How many valence electrons will nitrogen lose?
This Element Has An Atomic Number Of 7 And Belongs To Group 5.
The octet requires an atom to have 8 total electrons in order to have a full valence shell, therefore it needs to have a triple bond. Do you know the better answer? Determine how many of nitrogen’s electrons are classified as valence electrons.
The Valence Shell Is The Highest Energy Electron Shell An Atom Has.
The chemical element nitrogen has a total of 7 electrons. How many valence electrons are in an atom of nitrogen. It deserve to have either 3 or 5 valence electrons because it deserve to bond in the outer 2p and 2s orbitals.
The Last Shell Of Nitrogen Has Five Electrons.
The superscripts associated with these orbitals total to 6. How many valence electrons will nitrogen lose? 2 electrons are in shell one while there are 5 in shell two.
The Total Number Of Electrons Present In The Valence Shell Of An Atom Are Called Valence Electrons, And There Are A Total Of Five Electrons Present In The Valence Shell Of Nitrogen (2S22P3).
Nitrogen(n) is the 7th element in the periodic table. Asked by wiki @ 01/07/2021 in chemistry viewed by 27 persons. How many valence electrons does an atom of sulfur have.
Since The Last Shell Of A Rubidium Ion Has Eight Electrons, The Valence Electrons Of Rubidium Ion (Rb +) Are Eight.
Therefore, sulfur has 6 valence electrons. How many valence electrons are in a nh,ch ion? Nitrogen is in group 5, so it has 5 outer.
Related Posts
- 100 Micrograms Equals How Many Milligrams100 Micrograms Equals How Many Milligrams. The change of 1 mg ( milligram ) unit for a weight and mass measure equals = into 1,000.00 µg ( microgram ...
- Valence Electrons For FValence Electrons For F. Slnce the total number of electrons possible in s and p sublevels is elght, there can be no more than eight valence electron ...
- How Many Sides Do A Nonagon HaveHow Many Sides Do A Nonagon Have. This discussion on how many sides does decagon hasa8b10c6d4correct answer is option b. 5 do polygons have 7 sides?Q ...
- What Are Four Nitrogen Bases Found In DnaWhat Are Four Nitrogen Bases Found In Dna. Medical definition of nitrogenous base the nitrogenous bases in dna are adenine (a), guanine (g), thymine ...
- Ground State Electron Configuration Of NitrogenGround State Electron Configuration Of Nitrogen. The ground state electron configuration of ground state gaseous neutral nitrogen is [he]. Nitrogen a ...
- How Many Ounces Shredded Cheese In A CupHow Many Ounces Shredded Cheese In A Cup. It is a measure of volume. For instance, compute how many ounces or grams a cup of “monterey jack shredded ...
- How Many Electrons In An Atom Could Have N 2How Many Electrons In An Atom Could Have N 2. =2n=2 electronselectrons =5,ℓ=1n=5,ℓ=1 electronselectrons =7,ℓ=2, ℓ=−1n=7,ℓ=2,mℓ=− 1 electrons. That� ...




