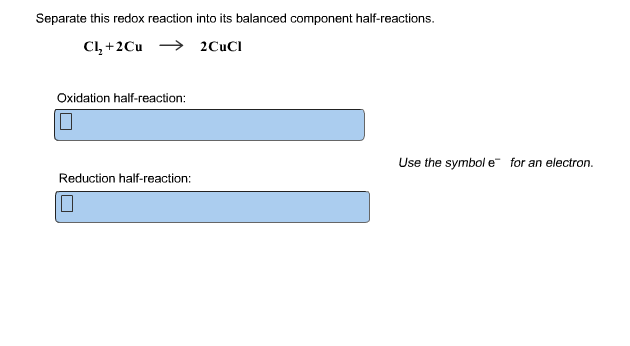
Separate This Redox Reaction Into Its Balanced Component Half Reactions Cl2 2Cu. Use the following steps to balance the redox reaction below: This problem has been solved!
I have no idea where to even begin. Use the symbol e− for an electron. While every initiative has been made to follow citation style rules, there may be part discrepancies.please describe the proper style manual or various other sources if girlfriend have any kind of questions.
Use The Symbol E− For An Electron.
Use the following steps to balance the redox reaction below: Use the symbol e for an electron. Cl2 + 2cu = 2cucl.
The Oxidation Number Of The Atom Gets Increased During This Reaction.
The oxidation refers to a species losing electrons. (× 4) reduction half reaction: Of cu in cuo = +2 o.s.
See The Answer See The Answer Done Loading.
Balance oxygen by adding h 2 o to the side. This problem has been solved! Separate the redox reaction into its component half‑reactions.
Learn This Topic By Watching Redox Reaction Concept Videos.
Use the symbol e− for an electron. Separate this redox reaction into its balanced component half‑reactions. Oxidation reaction is defined as the reaction in which an atom looses its electrons.
Of O In O 2 = 0 O.s.
Free unlimited access for 30 days,. Use the symbol e−e− for an electron. What is oxidation half reaction and reduction half reaction?
Related Posts
- Balanced Equation For Combustion Of OctaneBalanced Equation For Combustion Of Octane. The combustion of octane c8h18 follows this reaction. Write a balanced equation for the combustion of oct ...
- Fe2O3 Co Balanced EquationFe2O3 Co Balanced Equation. Given equation , fe2 o3 +. Balance the chemical equation algebraically:Balancing Redox Reactions CK12 Foundation from ck1 ...
- Pint Into LitresPint Into Litres. How to convert uk pints to litres? 5000 pints (uk) = 2841.31 liters:Pote de Vidro Pintado a Mão 3 Litros no Elo7 Vivendo from www.e ...
- Which Nims Component Includes The Incident Command System Ics AnswerWhich Nims Component Includes The Incident Command System Ics Answer. Furthermore, the national incident management system (nims) is a global, consis ...
- Half Pint How Many CupsHalf Pint How Many Cups. One gallon is equal to 4 quarts. 👍 here you can find how many cups [canada] is in half pint [uk], as well as, in any quanti ...
- Dont Let This Distract You MemeDont Let This Distract You Meme. Dont let this distract you from the fact that my name is rick harrison and this is my pawn shop. Add meme add image ...
- 172Cm Into Inches172Cm Into Inches. 1 foot is around 30.48 cm or 12 inches,. Convert 175 cm to other units.2.3 Inches To Millimeters Converter 2.3 in To mm Converter ...

