Pcl5 Formal Charge. Try to draw the pcl 5 lewis structure before watching the video. If, h= 2 = sp hybridization h= 3 = sp2 hybridization h= 4 = sp3 hybridization h= 5 = sp3d hybridization h= 6 = sp3d2 hybridization.
⇒ valence electrons of chlorine = 7 ⇒ lone pair electrons on chlorine = 6 The sum of formal charges on any molecule or ion results in the net overall charge. Here, v (valence electron of central atom) = 5 m ( monovalent atom) = cl = 3 as it is a neutral compound thus c and a will be 0
How To Determine If A Molecule Is Polar Or Nonpolar 12.
The formal charge of an atom in a molecule is the hypothetical charge the atom would have if we could redistribute the electrons in the bonds evenly between the atoms. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Formal charge is the actual charge on an individual atom within a larger molecule or polyatomic ion.
I Quickly Take You Through How To Draw The Lewis Structure Of Pcl5, Phosphorous Pentachloride.
Chloride obviously has a negative charge. Why is co2 nonpolar when co is polar 15. If we talk about the physical appearance of the compound, it is sensitive to water and.
If, H= 2 = Sp Hybridization H= 3 = Sp2 Hybridization H= 4 = Sp3 Hybridization H= 5 = Sp3D Hybridization H= 6 = Sp3D2 Hybridization.
In order to calculate the formal. Beginning with the terminal atoms, add enough electrons to each atom to give each atom. Let’s apply this formula to find the hybridization of pcl3.
C= Charge Of Cation A= Charge Of Anion.
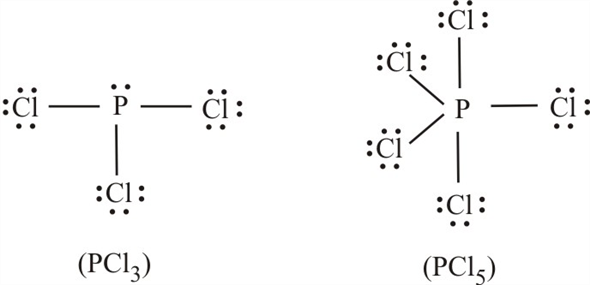
Place the bonding pair of electrons between each pair of adjacent atoms to give a single bond. I also go over formal charge. A phosphorus pentachloride molecule consists of 1 atom of phosphorus for 5 atoms of chlorine.
Here, V (Valence Electron Of Central Atom) = 5 M ( Monovalent Atom) = Cl = 3 As It Is A Neutral Compound Thus C And A Will Be 0
Solution for what is the formal charge on p in the molecule pcl5? This means that it can hold more than 8 valence electrons. Formal charge is determined for each atom in a molecule by:
Related Posts
- The Capacitor In The Figure Figure 1Begins To Charge After The Switch Closes At T 0SThe Capacitor In The Figure Figure 1Begins To Charge After The Switch Closes At T 0S. Find an expression for the current i at time t. Need solution t ...
- Nitrous Acid Formal ChargeNitrous Acid Formal Charge. The oxygen still has 8 valence electrons, it has an octet. Nitrous acid (molecular formula hno2) is a weak and monoprotic ...
- Pcl5 Compound NamePcl5 Compound Name. Although the molecular formula suggests the name tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of ...
