Bromine Electronegativity. Electro positivity is the exact opposite of electronegativity, therefore, we can say that cesium is the most electropositive element. It is located in group 17, the halogens.
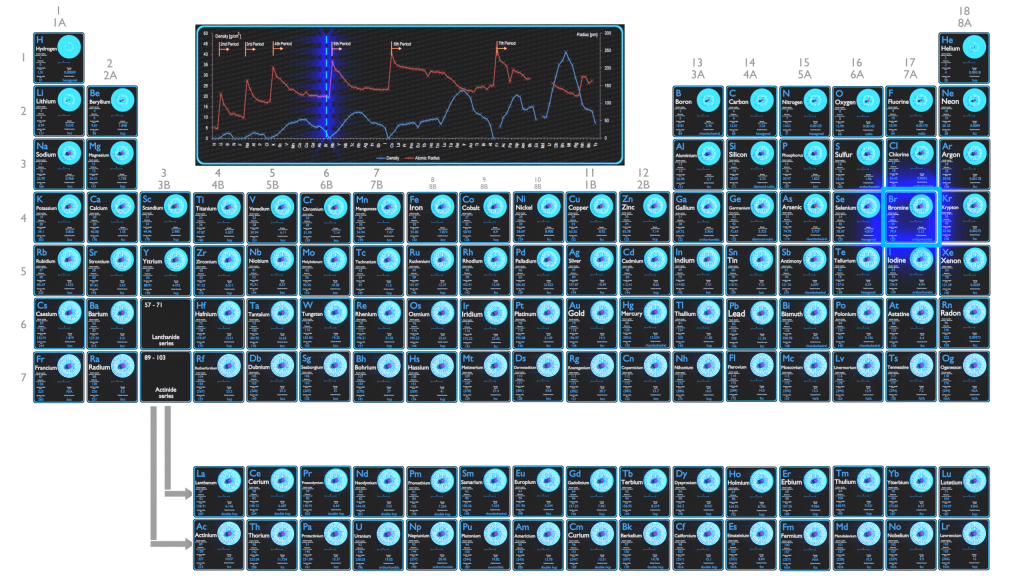
Therefore, it can never be placed in a central position because it is less prone to share electrons. The key difference between bromine and iodine is that bromine is in the liquid state at room temperature whereas iodine is in the solid state. Therefore, bromine is more electronegative than iodine.
Therefore, Both These Elements Have 7 Electrons In Their Outermost Electron Shell.
Electronegativity chlorine has an electronegativity of pauline scale 3.16. From the perspective of electronegativity, brf5 consists of several polar covalent bonds as the significant difference between the electronegativity of bromine is (2.96) and fluorine is (3.98) which is greater in the electronegativity by.5 (even though both of them are halogens). Electronegativity of bromine is 2.8 on mulliken's scale.
Electronegativity Of Bromine Electronegativity Of Bromine Is 2.96.
Halogens and thyroid gland thyroid health natural health supplements biochemistry. Every chemistry student in the known universe knows the general trends for electronegativity. Bromine has an electronegativity of pauline scale 2.96.
You Can Study The Detailed Comparison Between Bromine Vs Krypton With Most Reliable Information About Their Properties, Attributes, Facts, Uses Etc.
Electron affinity of bromine is 324.6 kj/mol. The electronegativity increases going from left to right across a period. It is located in group 17, the halogens.
Therefore, They Can Show Positive Oxidation States Like +1, +3, +5 And +7.
Electron affinity in chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Bromine and arsenic belong to the same period in the periodic table. Therefore, it can never be placed in a central position because it is less prone to share electrons.
You Can Compare Br Vs Kr On More Than 90 Properties Like Electronegativity , Oxidation State, Atomic Shells, Orbital Structure, Electronaffinity, Physical States, Electrical Conductivity And Many More.
Fluorine is the most electronegative element. 119 rows electronegativity is a chemical property which describes how well an. Electronegativity decreases going from top to bottom in a family.
Related Posts
- What Is Electronegativity Measured InWhat Is Electronegativity Measured In. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. If two bonde ...
- How To Find The Electronegativity Of An AtomHow To Find The Electronegativity Of An Atom. Subtract the smaller electronegativity from the larger one to find the difference. So, in reality, an e ...
