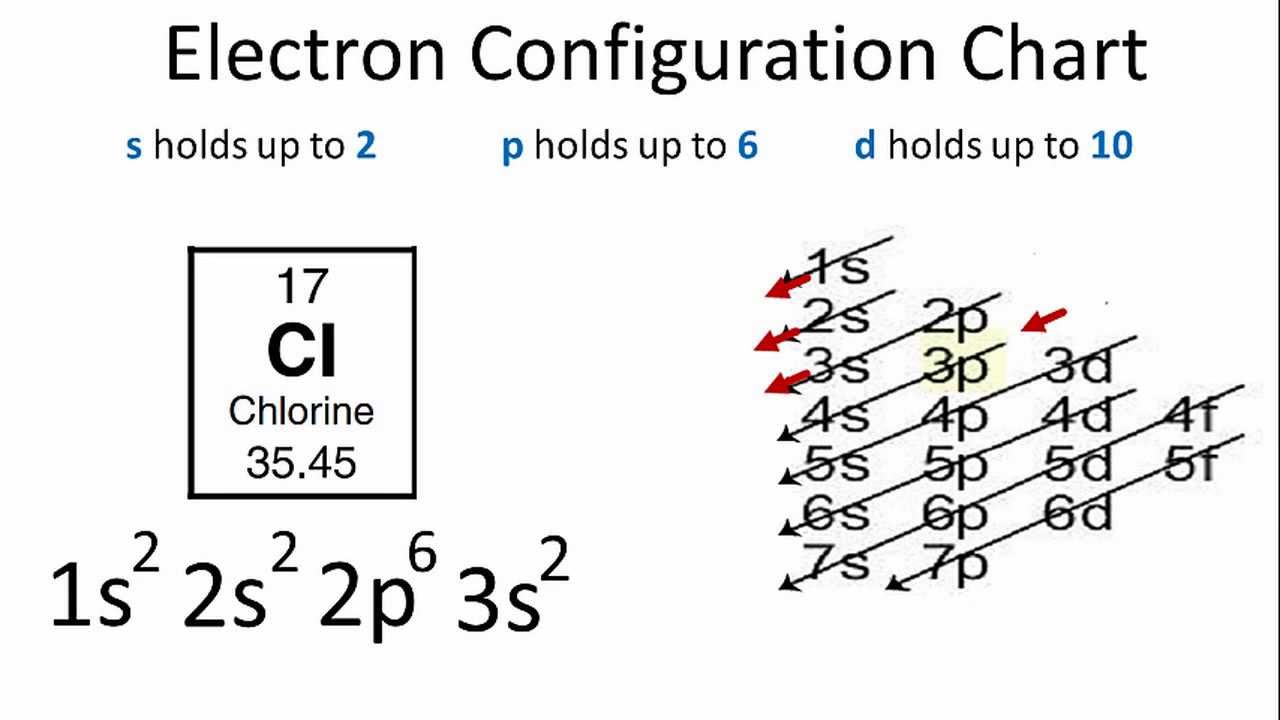
Enter The Full Electron Configuration For Cl. There is an electron in the last orbit of sodium(3s 1). Comments (0) answer & explanation.
Notice how the boxes are grouped by s, p, d f Add 1 electron (move 1 box to the right) notice how 1 more electron fills all the boxes on that row? (2) what is the atomic symbol for the noble gas that also has this electron configuration?
Cl−1S2 2S2 2P6 3S2 3P5 C L − 1 S 2 2 S 2 2 P 6 3 S 2 3 P 5.
Each paired up and down arrow indicates 2 electrons in that filled orbital. The k shell contains a 1s subshell hence it can carry 2 electrons, the l shell has 2s and 2p, and can carry 8 electrons. What is the atomic symbol for the noble gas that also has this electron configuration?
The Chlorine(Cl) Electron Configuration Will Be 1S2 2S2 2P6 3S2 3P5.
Each box represents 1 orbital. See the answer see the answer done loading. What is the atomic symbol for the noble gas that also has this electron configuration?
(2) What Is The Atomic Symbol For The Noble Gas That Also Has This Electron Configuration?
Enter the full electron configuration for. How many oxygen atoms are present in each of the formulas? Enter the full electron configuration for cl−.
Enter The Full Electron Configuration For Cr.
In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). If you don’t have a chart, you can still find the electron configuration. (1) enter the full electron configuration for cl− c l −.
A) V (Vanadium) B) 4S²:
Because the third energy level has eight electrons and is therefore full (3s 2 3p 6 ) it is called a noble gas. In writing the electron configuration for chlorine the first two electrons will go in the 1s orbital. In the case of chlorine the abbreviated electron configuration is ne 3s2 3p5.
Related Posts
- Fluorine Electron ConfigurationFluorine Electron Configuration. Fluorine atoms have 9 electrons and the shell structure is 2.7. The ground state electron configuration neutral fluo ...
- Ground State Electron Configuration ArsenicGround State Electron Configuration Arsenic. The ground state electron configuration of ground state gaseous neutral arsenic is [ar].3d 10.4s 2.4p 3 ...
- Halogen With The Least Negative Electron AffinityHalogen With The Least Negative Electron Affinity. Not all elements form stable negative ions in which case the electron affinity is zero or even pos ...
- H2O Electron Dot StructureH2O Electron Dot Structure. The o in h2o has 2 bond pairs and 2 lone pairs (again, 4 total pairs ). They also display the total number of lone pairs ...
- What Is The Abbreviated Electron Configuration For SilverWhat Is The Abbreviated Electron Configuration For Silver. Now, find the atomic number of the first. The energy of atomic orbitals increases as the p ...
- Write The Ground State Electron Configuration For The Chloride IonWrite The Ground State Electron Configuration For The Chloride Ion. (1) manganese(l) ion (ii) phosphorus atom (iii) chloride ion You may write either ...
- Cs2 Electron Pair GeometryCs2 Electron Pair Geometry. Here, the bond angles form an angle of 180 degrees. To determine the molecular geometry of a molecule, we need to get fam ...



