Bond Angle For Ch4. The standard explanation for why is that the lone pairs are larger than the hydrogens, creating repulsive forces that push the hydrogens down, decreasing the bond angle. Vsepr molecular shape (central atom):
The bond angle in nh3 is (smaller, larger) than the bond angle in ch4 because 1. The bond angles in trigonal planar molecules are larger than those in tetrahedral molecules. To apply the electronegativity argument, you should compare the distinct bond angles in c h x 2 f x 2 or in c h x 2 c l x 2.
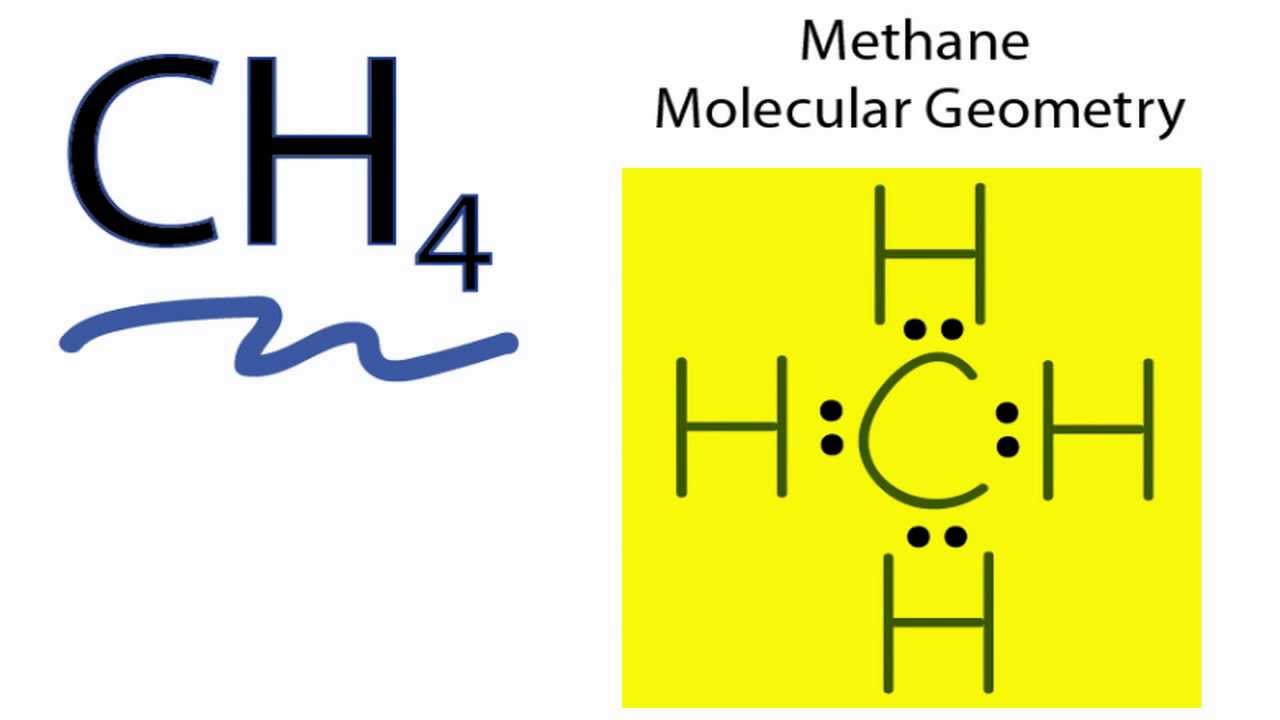
It Turns Out That Methane Is Tetrahedral, With 4 Equal Bond Angles Of 109.5° And 4 Equal Bond Lengths, And No Dipole Moment.
The central c atom in ch4 is attached to four h atoms through four sigma bonds. But in the case of nh3 and h2o molecules, their central atoms n and o have a lone pair of electrons. This lewis structure suggests that the optimal bond angle for methane is 90°.
Explain The Geometries And Bond Angles Of All The Molecules And Give A Reason For The Same.
• ch4, nh3, and h2o. 7 all six atoms of ethene lie on the same plane. Does ch4 have all equal bond angle?
The Lewis Structure For Each Molecule Show Us The Two Dimensional Arrangemen.
What is the angle between ch4? This angle has been measured experimentally and found to be 109.5°. Ch4 , nh3 , and h2o have same hybridization but different geometries and bond angles.
Consequently, What Is The Bond Angle Of C2H6?
The ideal bond angles are the angles that demonstrate the maximum angle where it would minimize repulsion, thus verifying the vsepr theory. Consider three molecules with tetrahedral electron domain geometries: The ch4 molecule will have 109.5° bond angles as there is no distortion in its shape.
Bond Angle Present In Methane (Ch4) Molecule Is:
109.5° what is the idealized bond angle? Figure 7.8 the shape of a methane molecule, ch4: The bond angle between h—c—h is 109.5° which is called the tetrahedral angle.
Related Posts
- What Is The Value Of The Bond Angles In Ch4What Is The Value Of The Bond Angles In Ch4. The bond angles in ch4 (109.5°), nh3 (107°) and h2o (104.5°) are different although these molecules have ...
- Find The Measure Of Each Angle Indicated AnswersFind The Measure Of Each Angle Indicated Answers. A) 35° b) 50° c) 59° d) 45° 3) 90° 40°? To find the equation of line we need the slope of the line ...
- Triple Covalent BondTriple Covalent Bond. The bond only pairs them. What is a triple covalent bond?How to distinguish between single, double and triple from www.quora.co ...
- Bond Angles Of Co32Bond Angles Of Co32. Bond angle is 109.5 degrees.it is equal in every bond a compound with only 2 atoms would not have a? Of the following species, a ...
- Bond Angle Of AmmoniaBond Angle Of Ammonia. Why is the bond angle in ammonia smaller than the bond angle in methane? The bond angle in ammonia is less than the standard 1 ...
- Bf3 Bond AngleBf3 Bond Angle. So bond angle in bf3 is 120° and in ncl3 it is 103°. Nh3 has a lone pair.ChemistryChemical Bonding Physical Chemistry Doubts from goi ...
- Define Angle Addition PostulateDefine Angle Addition Postulate. By the definition of supplementary angles, it is impossible to get the supplementary angle when we add two acute ang ...

