Bcl3 Lewis. Click to see full answer. Interhalogen molecules are those which contain two or more halogen atoms and no atoms of any other groups.
Therefore, option d is the right answer. Concept notes & videos 545. Why does bcl3 act as a lewis acid?
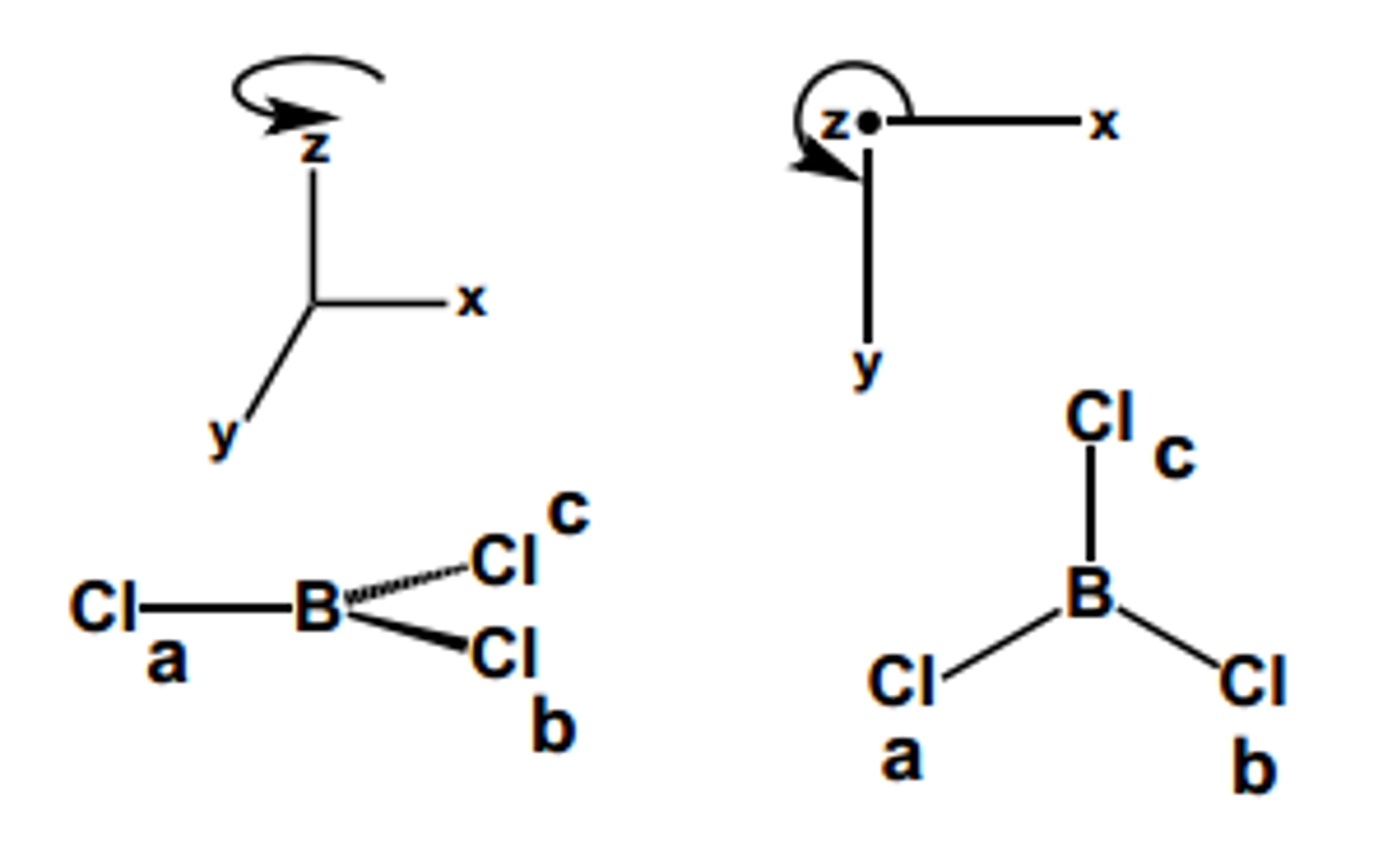
This Is A Trigonal Planar Arrangement And Implies That The Boron Must Be Sp2 Hybridized.
So a l is more electron deficient as compared to b hence a l c l x 3 must have higher lewis acid character. To complete its octet, it can accept an electron pair and hence acts as a lewis acid. We'll put boron at the center, and then let's put the chlorines around it, there are three of them.
Which Is Considered A Stronger Lewis Acid, Bf3 Or Bcl3?
The geometry of the bcl3 molecule can then be predicted using the valence shell electron pair repulsion theory (vsepr theory), which states that molecules will choose the bcl3 geometrical shape in which the electrons have from one another. At room temperature, this is a colorless gas that forms fumes and has a pungent odor. The central atom in bcl3 (that is boron, in trivalent state) has only six electrons around it — it is electron deficient and accepts electrons to complete it's octet.
The Extra Unhybridized P Orbital Is Empty, But Its Presence Is What Keeps The Three Sp2 Orbitals Separated By Exactly 120 Degrees.
Now, in bf3 molecule, b is small in size and thus can easily attract the incoming pair of electrons. Lewis acid are electron acceptors. Uncategorized ##”bcl”_3## is the stronger lewis acid.
Estructura De Lewis Estructura De Lewis Del Bcl3 (Tricloruro De Boro)
Click here👆to get an answer to your question ️ the lewis acid strength of bbr3, bcl3 and bf3 is in the order. Concept notes & videos 545. A number of the interhalogen compounds are unstable and are mostly covalent in nature.
Why Does Bcl3 Act As Lewis Acid?
Bcl3 is an electron deficient compounds since b atom has only six electrons in the valence shell. This has a cellular, trigonal planar geometry. Classify the following species into lewis acids and lewis bases.
Related Posts
- What Is The Lewis Dot Structure For Nh3What Is The Lewis Dot Structure For Nh3. Lewis dot diagram of nh3 the lewis structure of ammonia, nh3, would be three hydrogen atoms bonded to a nitr ...
- Lewis Structure Of Sulfur TrioxideLewis Structure Of Sulfur Trioxide. Drawing so3 lewis structure is very easy to by using the following method. So 3 has 24 valence electrons.ArchivoS ...
- Scl4F2 Lewis StructureScl4F2 Lewis Structure. Step 1 − step 3. Determine the electron geometry and molecular geometry of sif4.SCl4 Lewis Structure How to Draw the Lewis St ...
- Po33 Lewis Structure ResonancePo33 Lewis Structure Resonance. Mortality data was not reported. That's the second restyle group, and then the final.Image Showing Resonance Str ...
- Sef2 Lewis Dot StructureSef2 Lewis Dot Structure. It is important to look at what the lewis structure of sf2 is so that we can move ahead and look at other aspects of it. Th ...
- 03 Lewis Dot Structure03 Lewis Dot Structure. Let’s see how to draw lewis dot structure for nf3 with easy steps. Nocl, cf 2 cl 2, hcn;03 Lewis Structure from divinewsmedia ...
- Bcl3 Polar Or NonpolarBcl3 Polar Or Nonpolar. Therefore this molecule is nonpolar. The three chloride atoms have a negative charge, and the one boron in the.Is BCl3 a nonp ...




