How Many Valence Electrons Are In Br. Thus, we can conclude that there are seven valence electrons available for bonding in bromine (br). This means that bromine has seven electrons available for bonding whereas in order to completely fill.
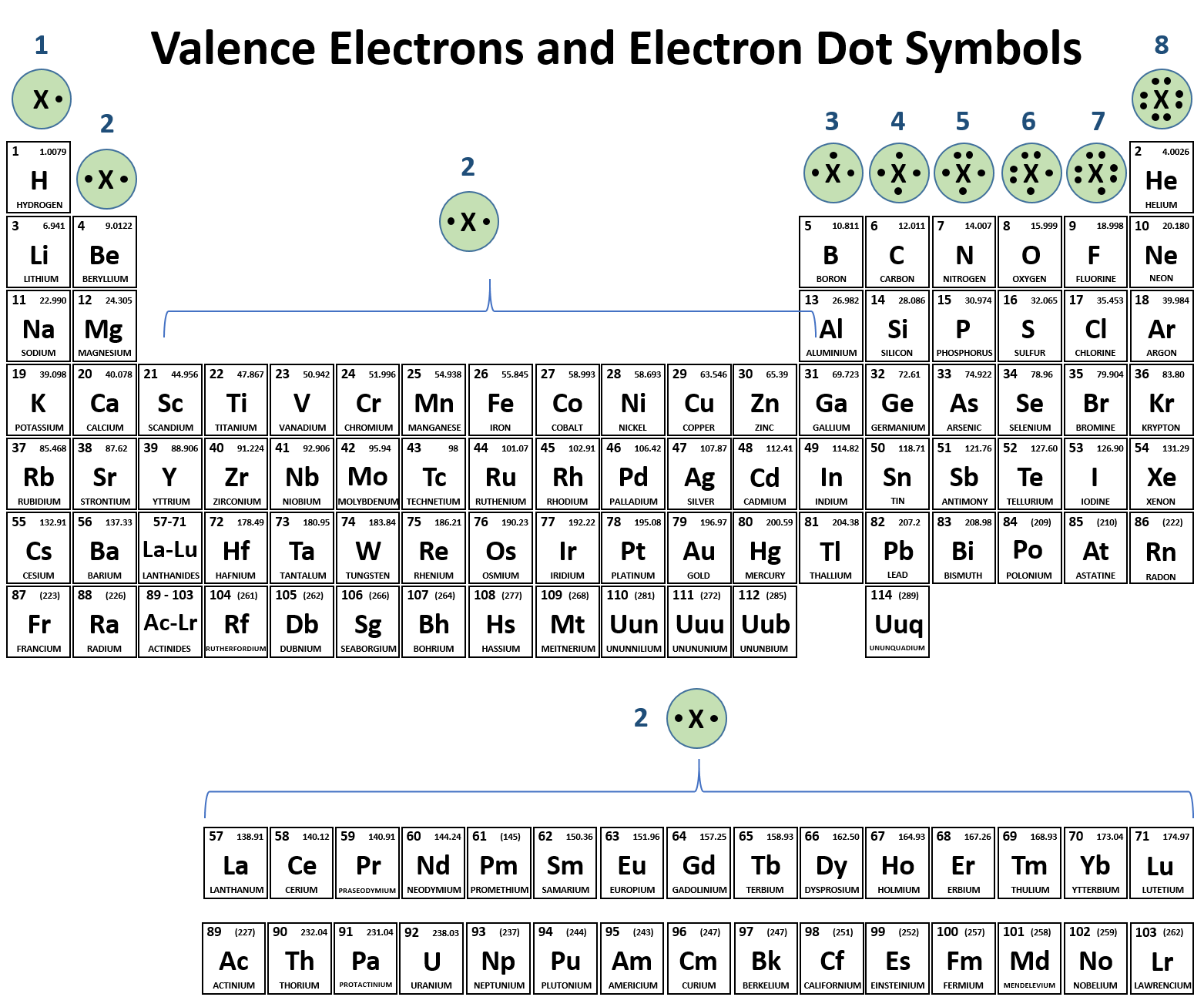
For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. Bromine has 28 core electrons.
Br 2 Is A Reddish Gas At Room Temperature.
Similar posts how many valence electrons does bromine(br) have? Thus, we can conclude that there are seven valence electrons available for bonding in bromine (br). This means that bromine has seven electrons available for bonding whereas in order to completely fill its octet bromine requires only one electron.
The Diagram Below Shows How A Bromine Atom Gains An Electron From The Element Lithium In Order To.
Bromine has an electron configuration of 1s22s22p63s23p64s23d104p5 the valence electrons are in the 4s and 4p orbitals giving bromine 7 valence electrons. Valence electrons in hydrogen (h) 1: •for atoms with 8 valence electrons, there is no change.
This Means A Bromine Atom Has Seven Valence Electrons.
Thus, bromine has seven valence electrons. Symbolically argon is denoted as ‘ar’ in the periodic table and its atomic number is 18. 5 valence electrons nitrogen has a total of 5 valence electrons, so doubling that, we would have a total of 10 valence electrons with two nitrogen atoms.
The Octet Requires An Atom To Have 8 Total Electrons In Order To Have A Full Valence Shell, Therefore It Needs To Have A Triple Bond.
The valence electrons of group 2a elements are in an s subshell.7. Learning check give the ionic charge for each of the following: No its not 35 that includes valence electrons.
Group 5A — The Pnictogens.
Bromine has 28 core electrons. Therefore bromine has 7 valance electrons. The first is to use the periodic table to figure out how many electrons bromine h.
Related Posts
- How Many Legs Under Table RiddleHow Many Legs Under Table Riddle. There are 4 table legs included in the answer, since the riddle doesn’t ask how many human legs are under the table ...
- Number Of Valence Electrons In OxygenNumber Of Valence Electrons In Oxygen. In o 3 molecule, there are three oxygen atoms. Since oxygen is in group 6 it means that it has 6 valence elect ...
- Valence Electrons For TinValence Electrons For Tin. 4 valence electrons tin has 4 valence electrons; 6 how many electron does gold have?Patterns of the Periodic Table Finding ...
- How Many Coulombs In A MicrocoulombHow Many Coulombs In A Microcoulomb. What is the value of 1 coulomb charge? The formula used in coulombs to microcoulombs conversion is 1 coulomb = 1 ...
- How Many Sides Does A Yield Sign HaveHow Many Sides Does A Yield Sign Have. How many square inches of material are needed to make a sign? You must wait until crossing vehi cles and pedes ...
- 180 Kilometers Is How Many Miles180 Kilometers Is How Many Miles. It is approximately equal to 0.621 miles, 1094 yards or 3281 feet. 180 degrees lines of latitude are called paralle ...
- 12 Meters Is How Many Feet12 Meters Is How Many Feet. 12 ft = 3.658 m. What do 12 meters mean in feet?I is for the idiosyncrasy of inches from www.rogerogreen.comVisit 12 mete ...




